Small, gas-phase clusters often exhibit novel electronic, magnetic, and catalytic properties compared to bulk materials having the same compositions. Such properties are strongly dependent on cluster size, even to the point where the addition or removal of a single atom can vastly alter a given property.
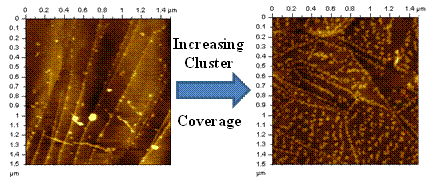
To harness their finite-size properties for potential applications in the macroscopic world, however, most clusters will either have to reside on surfaces or be incorporated into materials. For this reason, it is important to understand how size selected clusters behave on the surface of solid substrates. In these experiments gas phase clusters are first generated in the gas phase using a magnetron sputtering source before being size selected by passing them through a magnetic sector. The size selected clusters are subsequently soft landed onto a HOPG surface and imaged by either scanning tunneling or atomic force microscopy (STM or AFM). The AFM images (right) shows how the two-dimensional structure of size selected molybdenum oxide clusters deposited on HOPG vary as a function of cluster coverage. At comparatively low coverages the clusters preferentially nucleate at the HOPG step edge before forming larger aggregates on the HOPG terraces at higher cluster coverages. Future studies include plans to study the catalytic properties of size selected metal oxide clusters. These studies are conducted under the supervision of Professor Kit Bowen in the Chemistry Department at Johns Hopkins University.
 |
|---|
Influence of cluster coverage on the two-dimensional structure of size-selected molybdenum oxide clusters (resolved as lighter features) deposited on a HOPG substrate. |